![]()
Figure 1: Basic principle of SATIC method
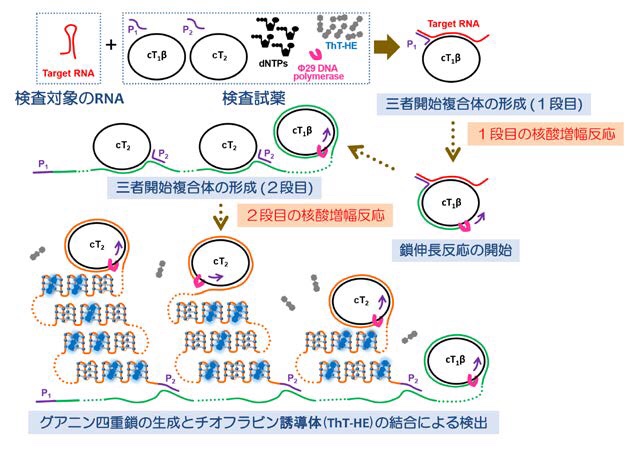
Figure 2: Flow to judgment (procedure)
Figure 3: Test results by PCR method
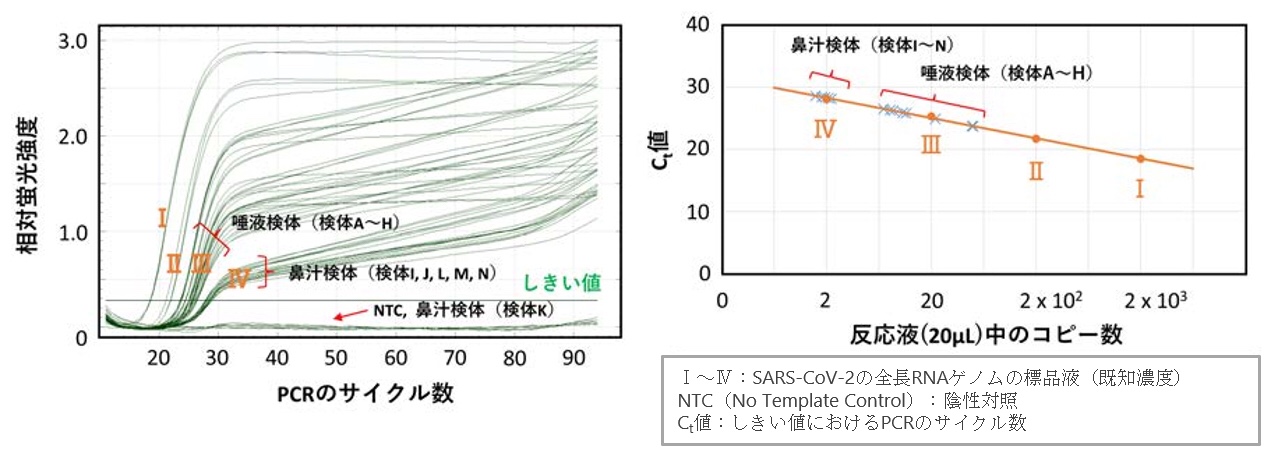
Figure 4: Test results by SATIC method

COVID-19: Shionogi,高灵敏度/快速病毒检测方法:在25分钟内检测唾液
盐野吉制药/日本大学/群马大学/东京医科大学
我们将与日本大学,群马大学和东京医科大学结成商业联盟,以快速诊断包括新冠状病毒在内的传染病领域的病毒。
从唾液等样品中
25分钟反应
无需检测设备
可以通过视觉判断
这是一种具有高灵敏度的快速诊断方法。
盐之木已与日本大学,群马大学和东京医科大学达成许可协议,就包括新型冠状病毒(SARS-CoV-2)在内的新型病毒快速诊断方法达成协议。
展望未来,我们将与公共机构,学术界和合作伙伴公司合作,将这种诊断方法投入实际应用。
常规检查方法:
诊断COVID-19患者的测试方法包括PCR方法(聚合酶链反应),该方法可检测从鼻腔,咽和唾液收集的标本中的病毒核酸,以及抗原检测试剂盒。
但是,这些测试方法仍然存在许多问题,例如需要专用的测量仪器,测量的简便性和速度以及在样本采集时医务人员感染的风险。
此检查方法:
由日本大学,群马大学和东京医科大学组成的合作研究小组已成功开发出了一种使用全新的创新核酸扩增方法(SATIC方法)的新型病毒快速诊断方法[1]。
SATIC方法是一种技术,不仅可以使用特异性高,灵敏度高的简单方法来测量特定基因,而且还可以测量突变基因以及体内分子(例如蛋白质和代谢产物)。
将SATIC方法应用于引起传染病的病毒的快速诊断方法具有以下所有特征。
核酸扩增法(SATIC法):
・无需检测设备即可通过肉眼轻松确定是否存在病毒感染
在样品采集后约25分钟内确定
没有非特异性反应,例如假阳性反应,并且具有相当于PCR方法的高灵敏度
从唾液,痰液和鼻咽拭子中检测
患者的侵入性较小,并且与样本收集相关的感染风险大大降低
新闻| Shionogi Pharmaceutical(Shionogi Pharmaceutical)
https://www.shionogi.com/jp/ja/news/2020/06/200622.html
Business Partnership with Nihon University, Gunma University, and Tokyo Medical University for a Rapid Diagnostic Methods for Viruses in the Field of Infectious Diseases, Including Novel Coronavirus
A Rapid Diagnostic Method with High Sensitivity that can be Visually Determined without the Need for a Detection Device in 25 Minutes of Reaction from Samples such as Saliva
Osaka, Japan, June 22, 2020 –
Shionogi & Co., Ltd.
announced today that Shionogi has agreed with
- Nihon University,
- Gunma University, and
- Tokyo Medical University on a license
agreement regarding a new rapid diagnostic method for viruses including the novel coronavirus (SARS-CoV-2).
We will work in collaboration with public institutions, academia including the above three universities, and partner companies to put this diagnostic method into practical use.
With continued social disruption caused by the worldwide spread of SARS-CoV-2, the spread of infection from not only patients with symptoms but also non-progressor and the infected person in the incubation period has become a major issue in the control of infectious disease.
At present, as the test methods for diagnosing patients with novel coronavirus infection (COVID-19),
PCR (polymerase chain reaction)
are used for diagnosing COVID-19 for detecting nucleic acids in viruses from samples collected from the nasal cavity, pharynx and saliva as well as antigen test kits.
However, many challenges remain with these testing methods, such as
- the need for specific detector,
- the ease and speed of measurement, and
- the risk of infection of healthcare workers
- during sample collection.
Therefore, there is a need for highly sensitive and inexpensive diagnostic methods that overcome these challenges.
The Joint Research Team,
has succeeded in developing a unprecedented innovative viral rapid diagnostic method using signal amplification by ternary initiation complexes method (SATIC method)[1].
The SATIC method is a technology
that can measure not only specific genes, but also mutant genes, as well as in-vivo molecules such as proteins and metabolites, using a simple method with high specificity.
This rapid diagnostic method using SATIC methods has all of the following features.
- The presence or absence of SARS-CoV-2 or influenza virus can be easily determined visually without a detector.
- Virus determination can be made in about 25 minutes after sample collection.
- There is no non-specific reaction such as false positive reaction, and high sensitivity equivalent to that of PCR.
- The ability to detect viruses from saliva and sputum as well as nasopharyngeal swabs reduces the invasiveness of the patient
- the risk of infection among healthcare workers associated with specimen collection as much as possible.
In the case of saliva, specimens can be collected by the patient themselves.
|News|Shionogi Co., Ltd.