东京大学:未分化人类iPS细胞培养:超高密度成功
-每个细胞的培养成本降低到 1/8-
东京大学研究生院
酒井康之教授
研究组
与钟化和日产化学合作
人类 iPS 细胞未分化增殖的成本,
我们开发了一个小规模的养殖系统,可以减少到 1/8。
小文化系统:
它有“被透析膜隔开的上下两个空间”,细胞培养在上部。
将生长因子储存在那里
营养物质和废物可以进出底部。
通过“在这里添加多糖”,生长密度可以比以前增加八倍。
iPS细胞生长因子的价格:
iPS 细胞在再生医学中备受期待。
然而,iPS细胞生长因子的价格很高。
那是。它是未分化细胞大量增殖的瓶颈。
透析膜培养方法:
但生长密度低,成本降低并未实现。
课题组新方法:
它的结构可以充分利用昂贵的生长因子。
细胞培养实验结果:
作为来自 iPS 细胞的“自组织能力的体现”
iPS细胞分泌的生长因子“Nordal”的浓度为
观察到随着培养而增加的现象。
这导致了大量的细胞培养。
诱导分化为器官细胞:
未来,我们将推动更高密度的细胞培养。
对于“诱导分化为器官细胞,花费数十倍”,
“检查此方法是否有效”。
已经不公开了
在“肝胰岛分化的第一阶段(内胚层分化)”
我们成功地“将使用的生长因子的数量减少到大约 1/8”。
TechCrunch 日本
https://jp.techcrunch.com/2021/11/24/tokyo-univ-ips-cell-high-density-cultivation/
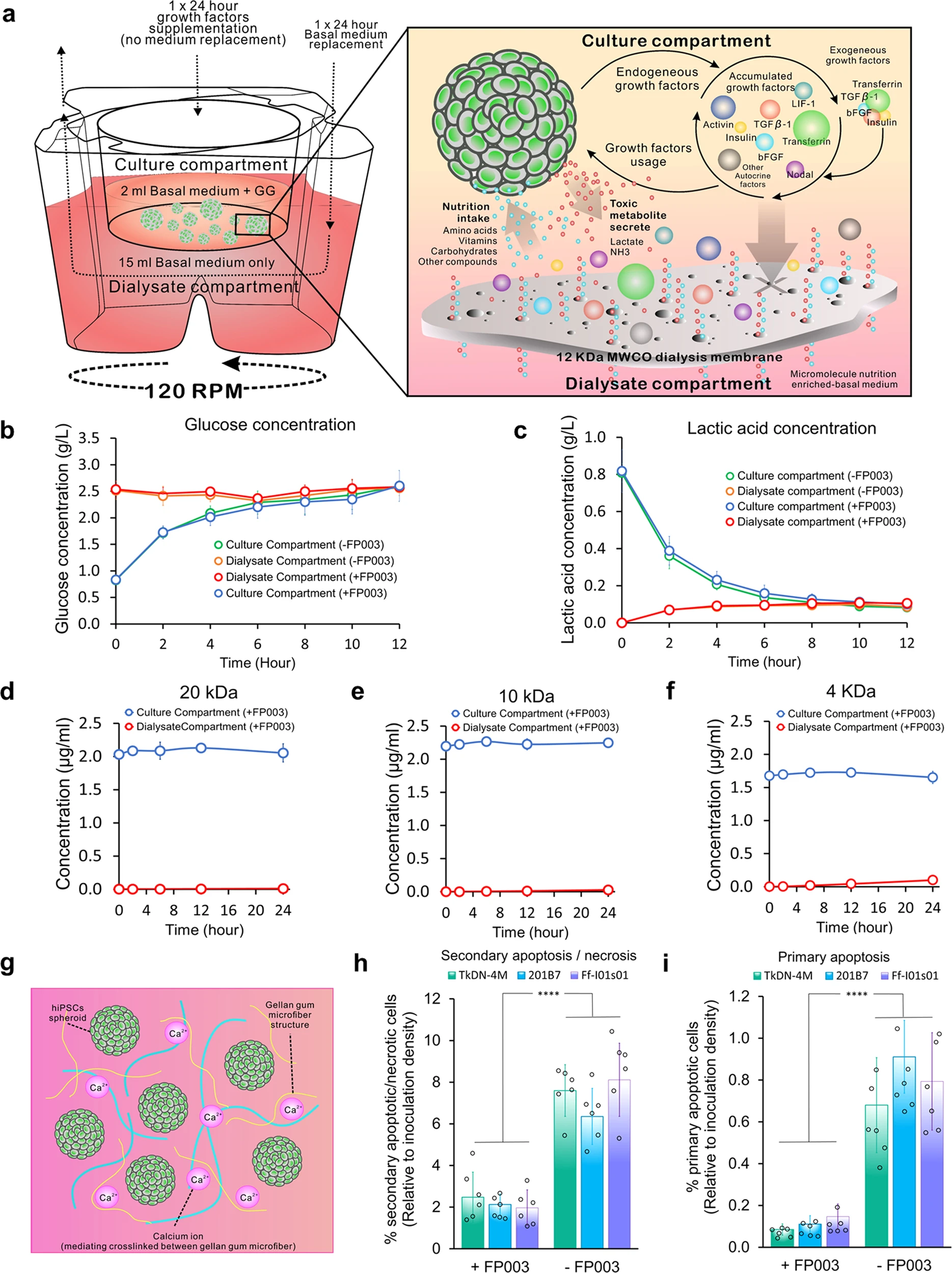
A miniature dialysis-culture device allows high-density human-induced pluripotent stem cells expansion from growth factor accumulation
Communications Biology
Abstract
Three-dimensional aggregate-suspension culture
is a potential biomanufacturing method to produce a large number of human induced pluripotent stem cells (hiPSCs);
however,
the use of expensive growth factors and method-induced mechanical stresspotentially result in inefficient production costs and difficulties in preserving pluripotency, respectively.
Here,
we developed a simple, miniaturized, dual-compartment dialysis-culture device based on a conventional membrane-culture insert with deep well plates.The device improved cell expansion up to approximately ~3.2 to 4×107 cells/mL.
The high-density expansion
was supported by reduction of excessive shear stress and agglomeration mediated by the addition of the functional polymer FP003.The results revealed accumulation of several growth factors,
including fibroblast growth factor 2 and insulin, along with endogenous Nodal, which acts as a substitute for depleted transforming growth factor-β1 in maintaining pluripotency.
Because we used the same growth-factor formulation per volume in the upper culture compartment,
the cost reduced in inverse proportional manner with the cell density.
We showed that
growth-factor-accumulation dynamics in a low-shear-stress environment successfully improved hiPSC proliferation, pluripotency, and differentiation potential.This miniaturised dialysis-culture system
demonstrated the feasibility of cost-effective mass production of hiPSCs in high-density culture.