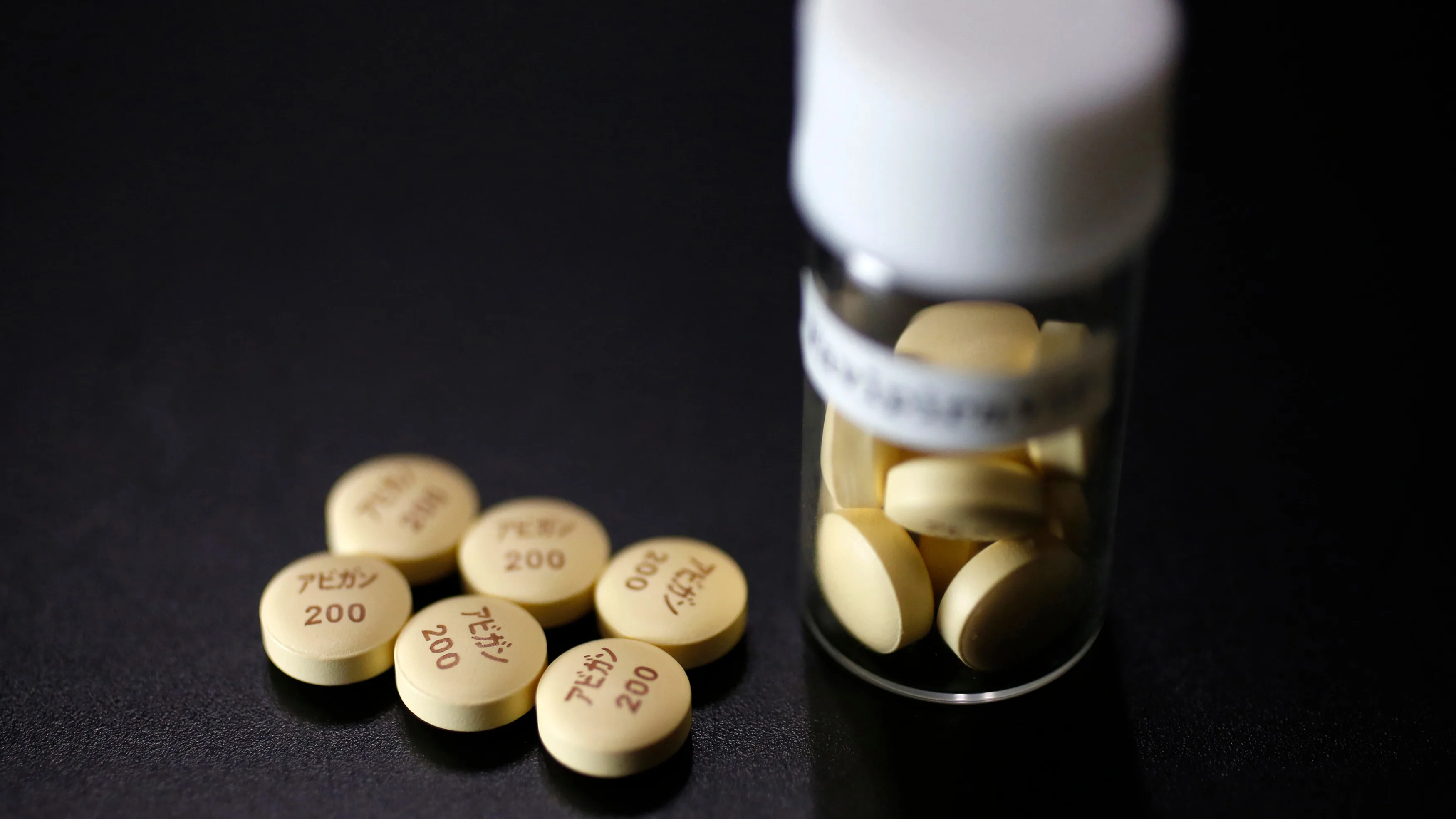
COVID-19:日本当局推迟批准Avigan:继续进行审议
-当局需要其他数据才能做出决定-
厚生劳动省:
富士富山化学正在申请AVIGAN批准作为一种新的冠状病毒感染治疗药物。
厚生劳动省在12月21日举行的理事会会议上忘记了对“ AVIGAN”的审查决定。
它宣布已决定再次继续进行审议。
继续审议的理由:
根据厚生劳动省的说法
从此时获得的数据很难清楚地判断其有效性。
在再次进行审议之前,它将等待公司的其他数据(例如正在进行的临床试验)。
彭博社
https://www.bloomberg.co.jp/news/articles/2020-12-21/QLOT8SDWRGG001
Japan health ministry delays decision on Avigan for coronavirus
TOKYO
The Japanese health ministry’s expert board on Monday
postponed a decision on whether to approve Fujifilm Holdings’ Avigan for coronavirus treatment, opting to examine more evidence to determine its efficacy.
“This is not a rejection of Avigan’s effectiveness,” a ministry representative said.
The board will continue
with its deliberations once additional clinical trial results come out, with a decision now expected no earlier than January.
Fujifilm called the ministry’s decision extremely regrettable.
the ministry’s board is believed to have taken issue with the fact
that Fujifilm ran a single-blind trial, in which doctors knew which patients received the drug as opposed to a placebo.
Single-blind trials
are considered less reliable than double-blind trials, where neither patients nor doctors know who receives the drug.
Nikkei Asia
https://asia.nikkei.com/Spotlight/Coronavirus/Japan-health-ministry-delays-decision-on-Avigan-for-coronavirus
Ministry to continue deliberation on Avigan | NHK WORLD-JAPAN News