人類 iPS 細胞:大量生產卵子和精子
・京都大學成功量產生殖細胞
・發表於英國科學期刊《自然》電子版 我們將為您提供在

Kyoto University research group:
Human iPS cells: mass production of eggs and sperm
A research group from Kyoto University announced this in the electronic version of the British scientific journal “Nature” on May 20th.

Mass production of germ cells:
Using human induced pluripotent stem cells (iPS cells), we succeeded in producing large quantities of germ cells, which are the source of eggs and sperm.
1. Germ cells are important cells that give rise to eggs and sperm.
2. The number of cells initially cultured can be increased by more than 10 billion times.
Elucidating the mechanism by which sperm and eggs are produced and the causes of infertility.
It is expected that this research result will help advance reproductive medicine.

Ethical discussion required:
Currently, the manipulation of fertilized eggs using iPS cells is prohibited under Japanese guidelines.
In the future, it is expected that discussions will take place from a variety of perspectives regarding the advancement of reproductive medical research.
1. It has not yet reached the stage where human eggs and sperm can be produced and used for reproduction.
2. There are technical and ethical issues, and there is still a long way to go in reproductive medicine applications.

Kyoto University Institute for Advanced Study: Research Group
Professor Michinori Saito of the Advanced Institute for Human Biology (WPI-ASHBi),
Yusuke Murase Special Researcher,
Members include Ryuta Yokokawa, a doctoral student.
https://scienceportal.jst.go.jp/gateway/clip/20240522_g01/#:~:text=ヒトの人工多能,増やすことができるという%E3%80%82

Kyoto University researchers :mass-generate cells capable of becoming human eggs
From NHK WORLD-JAPAN News
Researchers at Japan’s Kyoto University:
have developed a way to use human iPS cells to mass-generate cells capable of turning into human eggs.

A group:led by Professor Saitou Mitinori of Kyoto University’s Institute for Advanced Study
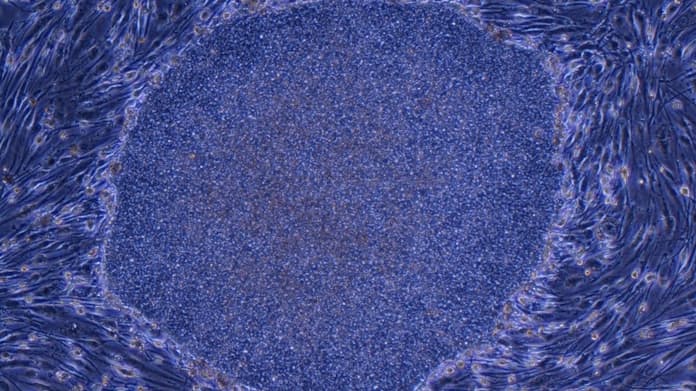
found a method to create base cells for reproductive cells from human iPS cells, and then change them into oogonia, which develop into ova.
The problem was that the amount of oogonia produced was too small.

producing huge amounts of oogonia:
When the researchers added a protein called BMP in culturing the base cells, they succeeded in producing huge amounts of oogonia.
The oogonia reportedly multiplied about ten billion-fold over roughly four months.
The group says:
it also successfully mass-generated, by the same methods, cells that develop into sperm.
Saitou said:
his team aims to apply the method to medical care, such as infertility treatment.
https://www3.nhk.or.jp/nhkworld/en/news/20240521_22/
Nature:In vitro reconstitution of epigenetic reprogramming
Abstract
Epigenetic reprogramming resets parental epigenetic memories and differentiates primordial germ cells (PGCs) into mitotic pro-spermatogonia or oogonia,
ensuring sexually dimorphic germ-cell development for totipotency 1.
In vitroreconstitution of epigenetic reprogramming in humans remains a fundamental challenge.
Here, we establish a robust strategy
for inducing epigenetic reprogramming and differentiation of pluripotent stem cell (PSC)-derived human PGC-like cells (hPGCLCs)
into mitotic pro-spermatogonia or oogonia, coupled with their extensive amplification (~>1010-fold).
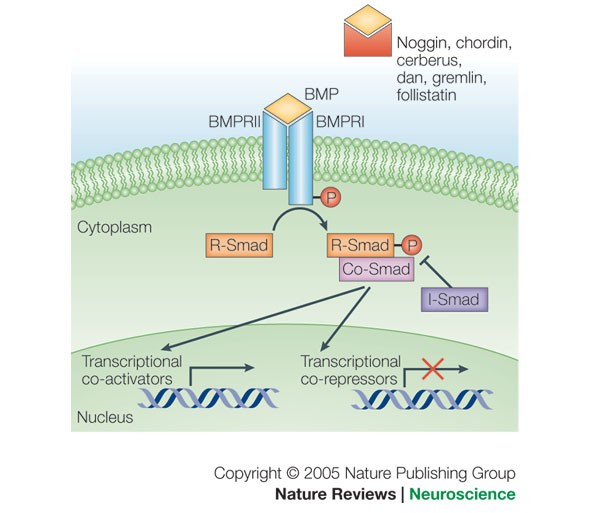
Strikingly, bone morphogenetic protein (BMP) signalling is a key driver of these processes:
BMP-driven hPGCLC differentiation
involves an attenuation of the mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/ERK) pathway and both de novo and maintenance DNA methyltransferase (DNMT) activities,
likely promoting replication-coupled, passive DNA demethylation.
On the other hand,
hPGCLCs deficient in tens-eleven translocation (TET) 1,
an active DNA demethylase abundant in human germ cells 2,3,
differentiate into extraembryonic cells, including amnion, with de-repression of key genes bearing bivalent promoters;
these cells fail to fully activate genes vital for spermatogenesis and oogenesis, with their promoters remaining methylated.
Our study elucidates the framework of epigenetic reprogramming in humans, making a fundamental advance in human biology,
https://www.nature.com/articles/s41586-024-07526-6